Author: Katherine Henderson / Editor: Jason Kendall / Reviewer: Thomas MacMahon, Josh Davison / Codes: A1 / Published: 19/11/2021
Context
Knowledge of valvular heart disease is important to an Emergency Physician in three main situations:
- Emergency physicians may hear a murmur during auscultation of the heart and need to be able to decide whether the murmur is significant or not.
- Patients with known valve problems may present with signs and symptoms suggesting that they are beginning to decompensate and need onward referral to prevent significant morbidity and mortality.
- Emergency physicians managing an acutely unwell patient with serious haemodynamic compromise should be able to recognise when a heart valve problem is involved.
The major valvular emergencies are:
- Infective endocarditis
- Papillary muscle rupture or flail mitral (posterior) leaflet due to ruptured chordae tendineae
- Prosthetic valve thrombosis / dehiscence
This article deals with the important presentations of valve disease in adults.
Definition
There are two cardiac valves on the left side (aortic and mitral) and two on the right side (pulmonary and tricuspid) (See Figure 1). Collectively the mitral and tricuspid valves are known as the atrioventricular (AV) valves. The four valves control the direction of blood flow through the heart. The valves open and close passively in response to changes in volume and pressure within the chambers. Closure of the tricuspid and mitral valves is helped by the papillary muscles which are attached to the cusps of the atrioventricular (AV) valves by the chordae tendineae . The papillary muscles act to stop the valves inverting or prolapsing.
Abnormalities of the papillary muscles or chordae tendineae can lead to valve incompetence. Valve dysfunction disrupts normal forward blood flow and places a haemodynamic burden on one or both ventricles. Picking up a murmur during auscultation of the heart is commonly the first step in diagnosing valvular heart disease.
Valvular stenosis (restricted opening) causes pressure overloading and valvular incompetence (failure of closure) causes volume overloading. The symptoms and signs of valvular heart disease can be understood by considering the effects of pressure and/or volume overloading on the heart and cardiovascular system as a whole.
Figure 1. Anatomy of the heart
Evaluating the patient with a murmur in the Emergency Department
A murmur is caused by turbulent blood flow. There are 3 main factors:
- Forward flow through a narrowed outlet
- Backwards or regurgitant flow through a leaking/ incompetent valve
- High blood flow in high output states such as anaemia, pregnancy, thyrotoxicosis, sepsis and fever
The presence of a murmur must be evaluated in light of the patients overall symptoms, signs and investigation results. Other signs of cardiac disease should be carefully sought.
Many murmurs in asymptomatic patients are innocent. Innocent murmurs are typically short systolic murmurs heard at the left sternal edge (LSE) which vary with respiration and which are decreased by sitting the patient up. There should be no other abnormal sounds or murmurs.
Classification and clinical approach to cardiac murmurs
A murmur is described in terms of:
- Where it is maximally heard,
- Where it radiates to,
- When in the cardiac cycle it comes ie. systolic, diastolic or continuous,
- The pitch ie. high, low or medium,
- The intensity.
Figure 2 suggests an ED-based approach to the evaluation of a murmur and Figure 3 shows a clinical classification of systolic murmurs. Intensity (see grading below) gives an indication of severity: systolic murmurs of Grade 3 or more are usually haemodynamically significant.
Learning Bite
Diastolic murmurs are nearly always pathological and require further investigation
Figure 2: An ED-based approach to the evaluation of a murmur
Intensity of a murmur the Freeman and Levine murmur loudness grading
- Grade 1 so faint that it can be heard only with special effort
- Grade 2 faint but can be heard easily
- Grade 3 moderately loud
- Grade 4 very loud
- Grade 5 extremely loud and can be heard with the stethoscope only just in contact with the skin.
- Grade 6 exceptionally loud and can be heard with the stethoscope just removed from contact with the chest
Figure 3: Classification of systolic murmurs
- MR mitral regurgitation
- TR tricuspid regurgitation
- AS aortic stenosis
- PS pulmonary stenosis
- HCM hypertrophic cardiomyopathy
- VSD Ventricular septal defect
There are two main forms of aortic valve disease:
- Aortic stenosis (AS)
- Aortic regurgitation (aortic incompetence) (AR)
Aortic stenosis
Aortic stenosis is defined as restricted opening of the valve cusps (see figure 4) causing an obstruction to left ventricular (LV) outflow. LV outflow obstruction can also be produced by a congenital abnormality above the valve (supra-valvular aortic stenosis) or by sub-valvular obstruction due to muscular hypertrophy as seen in hypertrophic cardiomyopathy. AS is severe once the valve area has decreased to 1cm2 or less (normal 3-4 cm2). The aetiology of AS is shown in Box 1.
Box 1: Aetiology of Aortic Stenosis
| Congenital | Acquired |
| Commonest cause in young adults. The valve can be bi- or unicuspid. In pure AS in the under 70s who require surgery 50% had a calcified bicuspid valve.(1) |
|
In the Cardiovascular Health Study (2) 26% of over 65s and 37% of over 75s had age-related degenerative calcific aortic valve thickening causing a systolic murmur but without significant obstruction (aortic sclerosis). 2% of over 65s and 2.6% of over 75s had significant aortic stenosis (See figure 5). The changes in the valves are similar to those seen in atherosclerotic vessels. Hypertension, smoking and raised cholesterol are all risk factors for aortic valve calcification.
Figure 4: Anatomy of the aortic valve
Figure 5: Calcific aortic stenosis
Pathophysiology
As the aortic valve area reduces a systolic pressure gradient develops between the left ventricle (LV) and the aorta. The progressive outflow obstruction requires the LV to contract more forcefully (increased myocardial contractility) to maintain stroke volume eventually resulting in LV hypertrophy. As long as the mitral valve is functioning normally the pulmonary circulation is protected and the patient may be asymptomatic for years. In severe disease the ventricle can no longer respond and LV function becomes abnormal. At this stage (i.e. critical aortic stenosis) the balance between cardiac output and myocardial muscle oxygen demand can be easily disrupted causing acute decompensation and severe pump failure.
Clinical features
The classic symptoms of aortic stenosis are:
- Breathlessness
- Chest pain
- Exertional syncope
Once symptoms occur prognosis is poor without surgical treatment. (3) Almost all patients with heart failure are dead within two years. In over 80% of patients who died of AS they had had symptoms for less than four years.(3) Sudden death is reported in 5-20% of patients but in fact is rarely truly sudden in that the patient has already developed symptoms even if not recognised as AS. Sudden death is a rare event in the truly asymptomatic patient (<1%/year (3)).
Breathlessness is usually the first symptom, followed by paroxysmal nocturnal dyspnoea, exertional syncope, angina and acute myocardial infarction. The sudden onset of atrial fibrillation (AF) may cause an acute progression of symptoms. GI bleeding from angiodysplasia may occur.
The clinical, ECG and CXR findings associated with AS are presented in Table 1.
Table 1: Clinical, ECG and CXR findings associated with aortic stenosis
| Pulse | Slow rising small volume with a sustained peak. (pulsus parvus et tardus) Often absent in the elderly due to loss of aortic compliance |
| Cardiac impulse | Sustained heaving apical impulse with a precordial thrill. Laterally displaced apex beat indicates onset of heart failure. |
| Auscultation | Harsh systolic ejection murmur 2nd intercostal space left sternal edge and radiating to the carotids The murmur softens and becomes prolonged as the severity of AS increases Single second heart sound (S2) in moderate AS Paradoxical splitting of S2 or soft/ obscured by murmur in severe AS. Fourth heart sound gallop rhythm |
| ECG | LVH criteria (or strain pattern) but may be absent despite severe obstruction: 10-15% of severe AS have normal ECGs May show RBBB or LBBB AF usually in association with simultaneous Mitral Valve Disease |
| CXR | Seldom helpful. May show normal sized heart and a dilated proximal ascending aorta. Late signs are of LV/LA dilatation and pulmonary oedema. Calcium in the aortic valve of a patient <45 is indicative of AS |
Management in the ED
Many patients will have known longstanding AS and be followed up regularly in outpatients but may present acutely with symptoms as a consequence of decompensation requiring treatment and admission.
Learning Bite
Any patient presenting with breathlessness, angina or syncope and found to have a systolic murmur will require careful workup and referral
Emergency measures
- Pulmonary oedema can be treated with diuretics and continuous positive airways pressure (CPAP) to reduce preload and improve ventilatory function. Nitrates and ACE inhibitors may cause a drop in after load and a significant drop in blood pressure and should be avoided.
- Recent excessive diuretics, vasodilator therapy or hypovolaemia may be responsible for the acute decompensation and require corrective measures.
- New onset AF can be treated with digoxin to slow ventricular response and improve stroke volume.
- Chest pain may be relieved by beta blockers which reduce myocardial oxygen demand and may improve coronary blood flow.
- Patients in heart failure are likely to need emergency surgery and have a higher operative mortality than the patients with AS who have preserved LV function. However they still have a better outcome from surgery than from medical management
- Up to 6% of older patients present in cardiogenic shock (5). These patients need aggressive medical therapy and emergency surgery.
Other management issues
All patients with AS need antibiotic cover for certain surgical procedures to protect against infective endocarditis.
Aortic Regurgitation / Incompetence
Aortic valve incompetence results from failure of the valve to prevent leakage (regurgitation) of some of the stroke volume back into the left ventricle from the aorta. It can occur because of aortic valve leaflet pathology or because of aortic root disease or a combination of these factors. Aortic regurgitation can be acute or chronic. The prevalence of AR in the Framingham study was 4.9%.The aetiology of AR is shown in Box 2.
Box 2: Aetiology of acute and chronic aortic regurgitation
| Acute AR | Chronic AR | |
|
Congenital | Acquired |
| Usually a bicuspid valve or supravalvular stenosis (suspect if isolated lesion in a chronic presentation) |
|
|
Pathophysiology
Regurgitation of blood into the left ventricle during diastole causes volume overloading. The pathophysiology of acute and chronic AR is different. In acute AR there is a sudden increase in the volume of blood in the LV during diastole. The left ventricle volume can only increase marginally in response to this acute change so left ventricular end diastolic pressure rises sharply. LA and pulmonary venous pressure rises and results in acute heart failure.
In chronic AR there is time for compensation and the LV progressively dilates and hypertrophies to maintain the ejection fraction. Tachycardia decreases the diastolic filling time and so reduces the regurgitant volume. During early stages of the disease the heart is able to respond to exertion with an appropriate increase in cardiac output. As a result AR can be tolerated for years.
Clinical features
Chronic aortic regurgitation
Patients may be asymptomatic for years although a murmur may have been previously noted.
Common symptoms:
- Awareness of the heart beat /palpitations especially at rest (because of the hyperactive dilated LV)
- Chest pain
- Fatigue
As the disease progresses:
- Heart failure
- Angina (as in AS this can occur despite normal coronary arteries)
Acute aortic regurgitation
In acute AR, the clinical presentation will depend on the underlying cause. If the regurgitation is mild the predominant symptoms may relate to the underlying cause; for example acute tearing chest pain radiating to the back suggests aortic dissection, or the peripheral signs and symptoms of sepsis in infective endocarditis. AR associated with aortic dissection means that the dissection involves the ascending aorta down to the annulus.
The clinical, ECG and CXR findings in acute and chronic AR are shown in Table 2.
Table 2: Clinical, ECG, CXR findings associated with acute and chronic aortic regurgitation
| Chronic AR | Acute AR | |
| Pulse | Rapid rise and quick collapse (water hammer pulse), double impulse, wide pulse pressure
|
Tachycardia rapid rate of rise of arterial pulse |
| Cardiac impulse | Hyperdynamic, maybe visible | Normal or hyperkinetic |
| Auscultation | Soft blowing diastolic murmur LSE. Best heard with the patient sitting forward in fully held expirationDuration of the murmur in diastole correlates with severity of AR
Austin Flint murmur apical diastolic murmur caused by obstruction of mitral flow produced by the partial closure of the mitral valve by the regurgitant jet and rapid rising LV diastolic pressure. May be seen in severe AR |
Early blowing diastolic murmur |
| ECG | In moderate/ severe disease- LVH with or without strain pattern | Non specific ST-T changes and sinus tachycardia or may be normal or show changes consistent with the underlying cause |
| CXR | Cardiomegaly with LV prominence and possibly dilated aorta | normal heart size and pulmonary oedema |
Learning Bite
If aortic regurgitation is sudden and severe the patient will present in acute pulmonary oedema or cardiogenic shock
Management in the ED
In acute severe AR secondary supportive management is needed while the underlying cause is being treated. Blood cultures should be taken unless there is an obvious underlying cause (e.g. aortic dissection or AMI)
Emergency measures
- Contact specialist services as may need surgical intervention as an emergency.
- In acute AR supportive measures are directed at reducing pulmonary venous pressure and increasing cardiac output. They will include the use of vasodilators, intubation and positive pressure ventilation.
- Inotropic support may be needed but can worsen the AR.
- Nitrates and diuretics have little effect and the intra-aortic balloon pump is contraindicated.
- Any patient with known AR presenting in heart failure will need admission for evaluation and consideration of aortic valve replacement (7).
- In an acute presentation of a patient with chronic AR adjustment of medical therapies such as diuretics, vasodilators, rate and rhythm control is needed acutely.
Other management issues
AR patients have an increased risk of developing endocarditis and should receive appropriate antibiotic prophylaxis.
There are three types of mitral valve dysfunction:
- Mitral Stenosis (MS)
- Mitral Regurgitation (MR)
- Mitral Valve prolapse (MVP)
Mitral stenosis
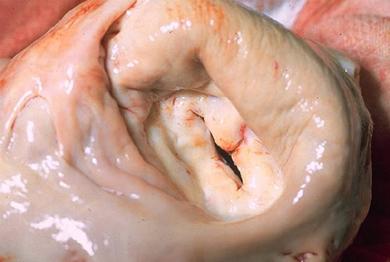
Mitral valve (see Figure 6) narrowing restricts the flow of blood from the left atrium into the ventricle impairing left ventricular filling. Symptoms may not occur until the valve area is reduced to 1-1.5 cm2 (normal 4-6 cm2) (See Figure 7). Once the valve area is <1 cm2 the patient is almost always has significant heart failure and very poor survival rates without surgery (8). The aetiology of MS is shown in Box 3.
Figure 6: Anatomy of the mitral valve
Figure 7: Marked mitral stenosis
Box 3: Aetiology of mitral stenosis
Acquired |
Other rare causes are: |
| Rheumatic heart disease (commonest cause worldwide) |
Left atrial myxoma can cause left atrial obstruction and mimic MS |
Pathophysiology
The obstruction to atrial emptying in MS causes an elevation in left atrial and pulmonary venous pressure, leading to reduced lung compliance and breathlessness on exertion. Reactive pulmonary arterial hypertension causes right ventricular hypertrophy and failure. Progressive stenosis cause left atrial dilatation and consequent atrial fibrillation which will further impair the function of the atrium. Left ventricular filling becomes impaired and cardiac output becomes compromised.
Clinical features
The main clinical presentations are:
- Exertional breathless, Orthopnoea, PND Breathlessness on exertion is often the first symptom noticed.
- Acute pulmonary oedema Hyperdynamic states with an associated tachycardia such as pregnancy, infection, uncontrolled AF and anaemia may result in a worsening of symptoms
- Atrial fibrillation Onset is associated with a marked deterioration of the patients clinical state. Risk of left atrial thrombus and systemic embolism
- Haemoptysis This used to be the second most common presentation but is rarer now that the disease is recognized sooner.
- Fatigue (due to reduced cardiac output)
Learning Bite
A patient may first present with symptomatic mitral stenosis when she becomes pregnant because of the hyperdynamic state of the circulation. Balloon valvuloplasty may be needed to get through the pregnancy.
The Clinical features, ECG and CXR findings associated with MS are presented in Table 3.
Table 3: Clinical, ECG and CXR findings associated with mitral stenosis
| Pulse | Small volume, irregular (usually AF) |
| Cardiac impulse | Tapping apex due to palpable first heart sound (S1) |
Auscultation |
Loud first heart sound (S1) (in sinus rhythm), Opening snap and rumbling mid-diastolic murmur. Early diastolic murmur of pulmonary regurgitation (Graham Steell murmur) |
ECG |
Broad or biphasic p wave best seen in Lead 2 indicating LA hypertrophy. R axis deviation. AF common, RV hypertrophy in later stages |
CXR |
Straightening of the left heart border indicating a dilated LA (double atrial shadow). Pulmonary congestion |
Other features |
Mitral facies: pink/purple patches on the cheeks reflecting reduced cardiac output and vasoconstriction |
Management in the ED
Any cause of tachycardia may result in an acute deterioration. Common causes are infection, new onset or uncontrolled atrial fibrillation, exercise or pregnancy. Symptoms will be improved by slowing the heart rate and allowing better ventricular filling during diastole. Atrial fibrillation can precipitate acute heart failure and may be difficult to treat.
Emergency measures
- Close attention to fluid balance.
- Antipyretics as appropriate.
- Find and treat underlying infection if suspected.
- Diuretics may be needed to relieve pulmonary congestion but addressing the shortened diastolic filling caused by any tachycardia will be of most benefit in the emergency setting.
- Rate control with beta blockers, digoxin or calcium channel blockers will be required for rapid atrial fibrillation. Any consideration of cardioversion must recognize the significant incidence of atrial thrombus and the risks of embolisation.
- Acute haemoptysis is relatively rare but can be severe. It is caused by vessel rupture due to venous congestion and may require referral to a cardiothoracic surgeon.
- All MS patients in atrial fibrillation should be on long term anticoagulants. There is little benefit to those in sinus rhythm. Systemic embolisation may be due to sub-therapeutic anticoagulation therapy. Patients may also present with complications of over anticoagulation.
Mitral regurgitation
Mitral regurgitation is back flow of blood from the left ventricle into the left atrium during systole. This causes volume overloading of the left atrium and an increased workload for the ventricle to maintain the ejection fraction. As with aortic valve incompetence mitral regurgitation may be acute or chronic. Mitral valve regurgitation occurs in about 2% of the population. Acute MR is a cardiovascular emergency. The aetiology of MR is shown in Box 4.
Box 4: Aetiology of acute and chronic mitral regurgitation
| Chronic MR | Acute MR |
heart disease or cardiomyopathy
|
Ruptured chordae tendineae or partial or complete papillary muscle rupture (e.g. due to acute myocardial infarction, trauma or infective endocarditis) |
Pathophysiology
During systole, a portion of the ejection fraction regurgitates into the left atrium. The portion is known as the regurgitant volume. This can also be expressed as the regurgitant fraction which is the regurgitant volume/ejection volume. Moderate MR is said to be present when the regurgitant fraction is in the range of 30 to 50%; severe MR is defined as a regurgitant fraction >50%.
In acute MR there is sudden volume and pressure overloading of the LA and pulmonary veins leading to acute pulmonary congestion. The LV stroke volume increases to maintain cardiac output but in acute myocardial infarction the ventricle may fail leading to cardiogenic shock.
Clinical features
Chronic Mitral Regurgitation
With progressive leaking of the mitral valve the left side of the heart has time to adapt. Both the LA and LV will enlarge to cope with the increase in blood volume and the LV will hypertrophy to deliver the increase in stroke volume needed to maintain cardiac output. Dilatation of the LA may result in AF and marked symptoms.
Acute Mitral Regurgitation
The patient will be acutely unwell with signs and symptoms of acute pulmonary oedema as well as signs of the underlying cause such as acute myocardial infarction or infective endocarditis. An echocardiograpy should be obtained urgently to rule out VSD, diagnose MR and assess LV function. The clinical features, ECG, CXR and ECHO findings associated with MR are shown in Table 4.
Table 4: Clinical, ECG and CXR findings associated with acute and chronic mitral regurgitation
| Acute MR | Chronic MR | |
| Pulse | Tachycardia | Tachycardia / AF common (Prominent a wave in JVP in SR) |
| Cardiac impulse | Hyperdynamic | Diffuse and displaced laterally. Systolic thrill at apex |
| Auscultation | Pansystolic murmur radiating to the axilla and back 3rd Heart Sound May be difficult to hear in the acutely breathless and tachycardic patient |
Pansystolic murmur radiating to the axilla and back 3rd Heart Sound |
| ECG | No changes or acute MI | LA and LV hypertrophy AF common |
| CXR | Pulmonary oedema with a normal sized heart or minimally enlarged LA | Increased LA and LV size Pulmonary venous congestion |
| ECHO | Urgent to rule out ventricular septal defect, diagnose MR and assess LV function |
Management in the ED
Acute MR associated with acute myocardial infarction has a poor prognosis. The 30 day mortality is 34% and 54% by 1 year. Acute papillary muscle rupture in acute myocardial infarction is associated with a 75% mortality without surgery within 24 hours.(9) MR in AMI may be due to posterior papillary muscle dysfunction with posterior wall infarction or dilatation of the mitral annulus with diffuse infarction of the LV or LV aneurysm formation. Rapid recognition of this complication of myocardial infarction is important. Blood cultures should be taken in any patient with acute MR and no obvious infarct.
Learning Bite
Acute MR associated with myocardial infarction is a cardiovascular emergency and may require surgical intervention
Emergency measures
- Contact specialist services as may need surgical intervention as an emergency.
- Treat acute myocardial infarction if underlying cause.
- Treat pulmonary oedema. This may be difficult if the patient is in cardiogenic shock. Intubation and positive pressure ventilation should be considered early. CPAP can be helpful. Reduce preload and afterload with nitrate infusion and ACE inhibitors if tolerated. Diuretics and inotropes may also be needed. Patients with cardiogenic shock with acute MR may benefit from intraaortic balloon pump.
Acute presentations of chronic MR are usually related to the onset of AF. Therapy is directed at reducing afterload to reduce LV work and controlling AF. Acute presentation of a patient with known chronic MR may indicate they require surgical intervention.
Mitral valve prolapse (Floppy mitral valve, Barlows syndrome)
MVP is prolapse of a portion of the valve leaflets into the left atrium during systole associated with a small amount of regurgitation of blood. The condition is found in between 2-5% of the population(10) and occurs more commonly in women. Most cases are idiopathic. It can be acquired secondary to IHD, Rheumatic heart disease and hypertrophic cardiomyopathy.
Pathophysiology
One or both of the mitral valve leaflets show fibromyxomatous changes. At the end of diastole the valve closes normally but as the pressure in the LV rises the leaflet proplases back into the LA. Strain on the papillary muscles can lead to mitral regurgitation.
Clinical features
Although most patients are asymptomatic, a wide variety of symptoms have been associated with MVP such as chest pain, breathlessness and palpitations. MVP may progress to clinically significant MR and there is an increased risk of infective endocarditis and cerebrovascular events. Men, those aged >45 and patients with significant MR are at high risk for complications. The association with sudden death is uncertain and is usually in the high risk group. Some patients have been noted to have long QT intervals. The clinical features, ECG and CXR findings are shown in Table 5.
Table 5: Clinical, ECG and CXR findings associated with mitral valve prolapse
| Pulse | normal |
Cardiac impulse |
normal |
Auscultation |
Midsystolic click a high pitched sound caused by sudden tensing of the mitral valve apparatus as the leaflet prolapse |
ECG |
Most cases no abnormality |
CXR |
No abnormality unless significant MR |
Acute problems
Attribution of symptoms to MVP is controversial. A patient with significant associated MR may have symptoms and signs related to this. Otherwise, when MVP has previously been diagnosed or is suspected, the role of emergency care is to exclude another acute cause for presenting symptoms. In the absence of another cause needing immediate treatment, the patient should be referred back to their own doctor for follow up or further investigation. Normal antibiotic prophylaxis precautions should be followed.
Both the tricuspid and pulmonary valves can be stenotic or regurgitant. In the emergency setting, the most important presentation is tricuspid regurgitation secondary to either infective endocarditis in intravenous drug users or chronic obstructive pulmonary disease (COPD) with pulmonary hypertension and subsequent right ventricular failure/dilatation.
Tricuspid regurgitation (TR)
The aetiology of TR is shown in Box 5.
Box 5: Aetiology of tricuspid regurgitation
| Congenital | Acquired |
| Ebsteins anomoly |
|
Clinical Features
Patients are usually asymptomatic unless right heart failure develops and the patient complains of oedema, ascites and abdominal pain from liver congestion. intravenous drug users may present acutely unwell with a staphylococcal endocarditis. The clinical, ECG and CXR findings associated with TR are shown in Table 6.
Table 6: Clinical, ECG and CXR findings associated with tricuspid regurgitation
| Pulse |
AF common Large v waves in JVP |
| Auscultation | Soft pansystolic murmur at LSE louder on inspiration Third heart sound (S3) often heard |
| ECG | No specific changes |
| CXR | Cardiomegaly, pleural effusion |
| Other features |
Tender enlarged pulsatile liver |
Acute problems
Tricuspid endocarditis in a drug abuser needs blood cultures and aggressive antibiotic therapy covering staphylococcal infection. Early surgery may be needed.
There are two main types of prosthetic valves
- Mechanical, non tissue valves
- Porcine, bovine or human tissue valves
Prosthetic valves can last decades but patients should be attending regular follow up to detect any deterioration which could then progress rapidly.
Acute problems with prosthetic valves
(i) Valve thrombus and embolisation:
Mechanical valves require life long anticoagulation; valve thrombosis is often associated with inadequate anticoagulation therapy. It is more common with mitral than aortic valves. Thrombotic obstruction of a tissue valve is rare.
A patient may present in cardiogenic shock, with systemic embolisation (cerebral infarction) or with sudden death. The diagnosis should be suspected if the patient is known to have a mechanical valve and the distinctive crisp click sound is reduced on auscultation. Echocardiography is needed to confirm the diagnosis. In a cerebral vascular event a CT scan should be performed to exclude a bleed.
Heparin anticoagulation or as necessary thrombolysis, thrombectomy or valve replacement.
(ii) Endocarditis:
This is most common in the first 2 months post valve surgery and usually is caused by wound infection or an intravenous line. Late infections are caused by the same organisms which infect native valves. All patients with prosthetic valves should have antibiotic prophylaxis.
(iii) Prosthetic valves and acute haemorrhage:
In acute haemorrhage the risk of causing valve thrombosis is outweighed by the risk of on going bleeding. Warfarin should be reversed (in consultation with Haematology services). When stable the patient should receive heparin and warfarin restarted.
Patients with existing heart valve abnormalities or prosthetic valves are at particular risk of developing infective endocarditis and these patients should be on prophylactic antibiotics. The British National Formulary contains comprehensive, evidence based recommendations.
Learning Bite
There should be a high index of suspicion of infective endocarditis in any unwell patient with a predisposing factor such as known valve disease, a prosthetic valve or intravenous drug abuse
Patients may present with clear cardiac manifestations or may be systemically unwell; endocarditis may only be diagnosed after careful investigation including multiple sets of blood cultures.
There may be symptoms and signs of infection, cardiac involvement and immune complex deposition as well as signs of septic emboli. Septic emboli may cause focal neurological deficits. The elderly may present with confusion. The classical signs of splinter haemorrhages, Oslers nodes, Janeway lesions and Roths spots are quite rare. Conjunctival or buccal petechial haemorrhages are quite common.
When the diagnosis is suspected antibiotic treatment should be discussed with local microbiologists prior to starting so that adequate samples are obtained.
Learning Bite
If infective endocarditis is suspected, 3-4 sets of blood cultures should be taken from different sites and at least 1 hour apart
Infective endocarditis is diagnosed by strict criteria (Duke classification) which entail characteristic positive blood cultures and evidence of cardiac involvement. A new cardiac murmur, progressive heart failure, AV block, palpitations or pericarditis can all be cardiac manifestations of infective endocarditis. Echocardiography should confirm presence of vegetations, new valve incompetence, a cardiac abscess or prosthetic valve dehiscence.
Patients with haemodynamic compromise should be discussed with the cardiovascular surgeons as a matter of urgency as immediate surgery may be indicated although mortality is high.
Antibiotic treatment should be determined by the sensitivities of the isolated organism; however, in the critically ill patient, they may need to be started before sensitivities are known and should follow microbiology advice.
Pitfall
An atrial myxoma may mimic infective endocarditis and present with non specific symptoms of fever, weight loss and embolic phenomena. There may be an associated murmur mimicking MS.
- Echocardiography is recommended for patients with heart murmurs and symptoms or signs of heart failure, myocardial ischaemia/infarction, syncope, thrombo-embolism, infective endocarditis, or other clinical evidence of structural heart disease. (Grade of evidence 1c)
- Echocardiography is not recommended for patients who have a grade 2 or softer midsystolic murmur identified as innocent or functional by an experienced observer. (Grade of evidence 3c)
- A patient presenting with heart failure, angina or syncope who has the clinical signs of aortic stenosis needs emergency referral for evaluation.
- Acute aortic regurgitation is a cardiovascular emergency. Important causes to consider are infective endocarditis, aortic dissection, rupture of an aortic valve leaflet (e.g. trauma) and prosthetic valve dysfunction.
- Rupture of a papillary muscle necessitates urgent surgical treatment after stabilization of the haemodynamic status, using an intra-aortic balloon pump and vasodilators. (European Society of Cardiology Guidelines 200711)
- There should be a high index of suspicion of infective endocarditis in any unwell patient with a predisposing factor such as known valve disease, a prosthetic valve or intravenous drug abuse particularly if there is a murmur suggesting valve incompetence.
- If infective endocarditis is suspected 3-4 sets of blood cultures should be taken from different sites and at least 1 hour apart.
- Passik CS, Ackermann DM, Pluth JR, Edwards WD. Temporal changes in the causes of aortic stenosis: a surgical pathologic study of 646 cases. Mayo Clin Proc. 1987 Feb;62(2):119-23.
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997 Mar 1;29(3):630-4.
- Baumgartner H. Aortic stenosis: medical and surgical management. Heart. 2005 Nov;91(11):1483-8.
- Mullany CJ, Elveback LR, Frye RL, Pluth JR, Edwards WD, Orszulak TA, Nassef LA Jr, Riner RE, Danielson GK. Coronary artery disease and its management: influence on survival in patients undergoing aortic valve replacement. J Am Coll Cardiol. 1987 Jul;10(1):66-72.
- Otto CM, Mickel MC, Kennedy JW, Alderman EL, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation. 1994 Feb;89(2):642-50.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA. 2000 Apr 5;283(13):1703-9.
- Chaliki HP, Mohty D, Avierinos JF, Scott CG, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002 Nov 19;106(21):2687-93.
- OLESEN KH. The natural history of 271 patients with mitral stenosis under medical treatment. Br Heart J. 1962 May;24(3):349-57.
- Tcheng JE, Jackman JD Jr, Nelson CL, Gardner LH, et al. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992 Jul 1;117(1):18-24.
- Freed LA, Benjamin EJ, Levy D, Larson MG, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002 Oct 2;40(7):1298-304.
Useful Guidelines
- Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A; Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007 Jan;28(2):230-68.
- Habib G, Hoen B, Tornos P, Thuny F, et al. Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009 Oct;30(19):2369-413.