Author: Hadassah Ihlenfeldt, David Quinn, Riad Hosein / Editor: Lauren Fraser / Codes: / Published: 25/06/2021
Patient knowledge
A partially implantable electrically driven pump that drains blood from the LV apex and returns it to the aorta (usually ascending) powered by a subcutaneously tunnelled driveline cable to portable batteries or mains power supply. It supplies a continuous flow of blood from the left ventricle to the aorta, independent of left ventricular function, therefore taking over the work of the left ventricle.

Your patients should know more about their LVAD than you do.
Your patient and their family should be able to:
- Care for the external components
- Change the driveline dressings in a sterile way
- Respond to alarms and emergencies
- Change the power source
- Know when to contact the VAD hotline
Indications and Aims
| Indications | Aims |
| Bridge to transplant to allow survival until a donor heart becomes available | Increase cardiac output and end organ perfusion |
| Bridge to candidacy- for patients unsuitable for transplant due to a condition that could be reversed by an LVAD. For example, to improve end organ dysfunction such as renal failure and pulmonary hypertension which if improved, would allow them to be a candidate for heart transplantation. | Improve symptoms of heart failure and reduce hospitalisation |
| Destination therapy in patients unsuitable for transplant for example, due to significant comorbidities or advanced age. | To relieve congestion |
| Improve survival and quality of life in end stage heart failure |
Now you know why an LVAD is implanted, can anyone have one? – No not everyone.
The contraindications to LVAD therapy:
- Some complex congenital heart disease the anatomy of the heart needs to allow for an LVAD to be implanted.
- Restrictive cardiomyopathy with small LV capacity The LVAD relies on filling of the left ventricle.
- Significant primary lung disease
- Severe peripheral vascular disease The LVAD function will be affected by systemic vascular resistance.
- Irreversible neurological deficit
- Active acute infection
- Active bleeding LVAD patients need anticoagulation
- Recent malignancy with anticipated survival <2years
- Psychosocial issues care of the LVAD and response to alarms requires a patient who is compliant and able to care for the LVAD
- Poor RV function The LVAD relies on left ventricular filling and therefore RV function.
Epidemiology
Now we understand what an LVAD is, how commonly are they used?
There are currently around 300 patients in England living with Ventricular Assist Devices. This number is fluctuant as the patients go onto heart transplants, or unfortunately die.
There are 6 centres in England that are licenced to implant LVADs: Birmingham Queen Elizabeth Hospital, Harefield Hospital, Manchester Wythenshawe Hospital, Newcastle Freeman hospital, Royal Papworth hospital, and Great Ormond Street Hospital.
During 2018-2019:
- 102 long term VADs inserted into adult patients. This number has remained relatively stable for the last 3 years.
- 29 VADs inserted into 20 paediatric patients.
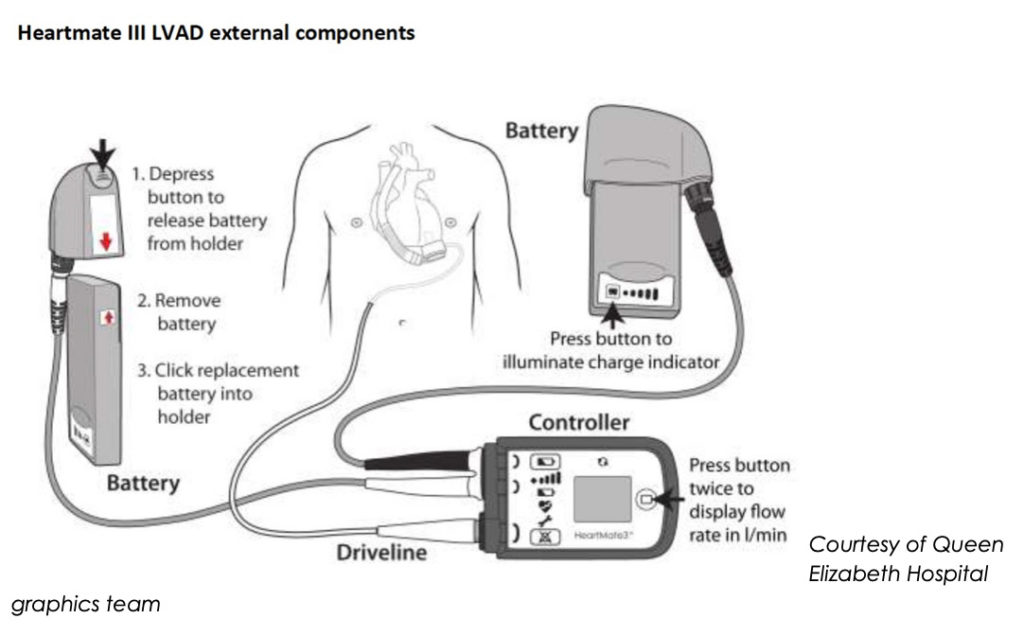
Components


Courtesy of Queen Elizabeth Hospital graphics team
Physiology
Learning Bite
Remember, there is often no palpable pulse
- The rotor pump imparts kinetic energy to the blood driving it through the device and into the aorta via the outflow graft
- This provides a continuous flow throughout the cardiac cycle
This flow is afterload sensitive - (Near) completely offloads the left ventricle ejection
- Flow is dependent on LV filling, itself dependent on volume status, right ventricular function and pulmonary vascular resistance
LVADs currently in use
There are a number of device makes and models that you may encounter.
The most common devices are the Heartware and the Heartmate III. Both magnetically levitated radial continuous flow, non-pulsatile devices.
There are other devices including the Jarvic2000 and the Heartmate II, which are continuous flow non-pulsatile axial impellar pumps.
The pictures below highlight the LVADs you are likely to see in the emergency department.
Notice they have 2 battery sources but only 1 is required for the LVAD to function. If both batteries are off, you have 15 minutes before the controller based power supply fails.
This should give time for the controller to be plugged into a mains power source or a fresh battery connected.
There are lights on the battery to show how well charged it is.

Patient assessment
Temperature, GCS and respiratory rate are all measured for LVAD patients as we would for any other patient.
Differences:
- No pulse due to continuous flow. (Some patients may retain a pulse depending on LVAD type and underlying ventricular ejection). The fully offloaded LV has no pulse because it is empty and the systemic circulation is driven by a continuous flow pump.
- Unreliable oxygen saturations: a pulse oximeter relies on pulsatile flow.
- Blood pressure taken using Doppler sonography to measure a MAP (normal 60-90 mmHg) using a blood pressure cuff using Doppler to either listen or with ultrasound to see at what pressure flow begins would indicate the MAP.
- No Heart sounds. Instead, you will hear a continuous hum
Go back to your basics of patient assessment:
Capillary refill time, temperature of peripheries, neurological status, urine output.
Here is a link to a video showing how we can measure the blood pressure of an LVAD patient.
Normal parameters of an LVAD
Pump Speed: (in rpm) this is programmable
- 5000-6000 rpm for Heartmate III
- 8000-10000 rpm for Heartmate II
Pump flow: (L/min) this is a calculated value
- Derived from speed and power consumption and not directly measured.
- Flow calculation is proportional to power consumption flow may not actually represent output to the patient.
Power consumption in watts
Pulsatility index
- An indication of background native ventricular function
With all VAD patients, you will need to call the VAD hotline early as you will need specialist input from an early stage.
There are many complications and considerations with VAD patients, we are only going to cover those that we have some input into as ED clinicians.
Arrhythmias
Atrial Fibrillation
Common and may result in haemodynamic instability and like in normal patients may be well tolerated or result in haemodynamic instability
Ventricular arrhythmias: (VT and VF)
VT is common and again may be well tolerated.
VF is often a terminal event but a small percentage of patients can tolerate it these patients may be alert and talking.
Ventricular arrhythmias may be due to:
- Underlying scar
- Suction events (Mechanical sucking down of left ventricle from inflow cannula) related to reduced flow to LV such as in RV failure, hypovolaemia, elevated PA pressures
- Dilating RV
Ventricular arrhythmias are better tolerated in LVAD patients due to the physiological support provided by the VAD.
Persistent arrhythmias or repeated RV fibrillation can compromise RV function remember the importance of RV function on VAD mechanics.
Learning bite
The LVAD is only as good as your RV.
Arrhythmia top tips
- Call the VAD hotline early and involve transplant cardiologist early
- Non- sustained ventricular arrhythmias do not need emergency management
- Amiodarone is generally the drug of choice for arrhythmia management. This will only be needed in the stable sustained arrhythmia patient. These patients can be transferred to the VAD centre before giving amiodarone, however if you have a prolonged transfer time, you may have to give amiodarone after speaking with the VAD hotline to prevent a sustained arrhythmia causing right ventricular dysfunction.
- Cardioversion under sedation is safe preferably done in a VAD centre.
Hypotension (Map <60mmHg)
Causes of low blood pressure in an LVAD patient are split into flows increased and flows decreased:
You can see if the flows are increased or decreased by looking at the controller panel. This will tell you the flow rate and alarm if abnormal.

Bleeding
This is a common cause of morbidity and mortality in LVAD patients.
20-40% of patients will have a bleeding event requiring intervention.2
Bleeding is multifactorial, and due to:
- Anticoagulation
- Platelet dysfunction
- Lysis of Von Willibrand Factor
- RV dysfunction causing hepatic congestion and failure
- Vasoplegia
- Mucosal vascular dysplasia


Gastro-intestinal Bleeding – Key learning points:
- Small bleeds can turn very quickly into large life-threatening bleeds due to degree of coagulopathy. Endoscopy is indicated urgently in these patients.
- Urgent endoscopy in these patients will also allow reversal of anticoagulation for smallest possible period of time as reversing the anticoagulation puts these patients at risk of pump thrombosis and failure.
- Reversal of anticoagulation is a multi-disciplinary decision Haematology, cardiac surgery and gastroenterology all need to see the patient.
Stroke
Very high risk of stroke 10%3
Highest risk in the first 6 months post implant
Early CT head and CT angiography is needed when there is any suspicion of stroke.
Learning bite
You Cannot MRI LVAD patients
Ischaemic:
- Early consideration of thrombolysis systemic or targeted intra-arterial. This needs to be a stroke specialist decision.
- Consider thrombectomy
Haemorrhagic:
- Reversal of anticoagulation with prothrombin complex concentrate Again an MDT decision utilising the VAD specialists and haematologists
Infection
Affects 14-35%4 of VAD patients and is a significant cause of morbidity and mortality.
Key points:
Driveline infections are common and easy to spot. If the driveline looks ok, think of either a deep driveline infection or VAD-related non-driveline infection or a non-VAD related infection.
The table below summarises some of the salient points and actions to take when suspecting infection.
Classified into 3 categories:

Follow your standard trauma care and ATLS protocol as for a non VAD patient.
Remember you cannot rely on feeling a pulse, or measuring a BP or oxygen saturation trace You need to go back to your basic patient assessment.
Inform your VAD centre and your Cardiac transplant surgeon at your local centre as soon as possible.
Aim for early invasive BP monitoring via an A-line this will give you an accurate MAP and allow you to monitor the trend.
Order specific xrays to assess for pump position and driveline integrity (AP chest xray and a targeted driveline xray).
Use CT and CT angiography as normal for a non-VAD patient
Learning bite
You cannot MRI these patients
Below are the 3 LVAD emergency algorithms published by Bowles, CT et al.5
These are a clear guide to follow in a collapse or sudden deterioration in an LVAD patient.
No one will be expected to memorise these but these should be read out in a step by step manner to make sure the correct process is followed.



- NHS England, Annual report on mechanical circulatory support related to heart transplantation, Report for 2018/2019, Page 11.
- Leebank F.W.G, Muslem R., Bleeding in critical care associated with left ventricular assist devices; pathophysiology, symptoms, and management. Hematology Am Soc Hematol Educ Program 2019; 2019 (1) 88-96.
- Deepak, A, et al., INTERMACS analysis of stroke during support with continuous flow left ventricular assist devices: Risk factors and outcomes. JACC heart fail, 2017, Oct 5: 703-711.
- Radoslav, Z, et al., In full flow: Left ventricular assist device infections in the modern era. Open Forum Infectious diseases, Volume 7, Issue 5, May 2020.
- Bowles, C, et al., Algorithms to guide ambulance clinicians in the management of emergencies in patients with implanted rotary left ventricular assist devices. Emergency Medicine Journal. 2017. Volume 34, Issue 12: 842-850
- Lim, Howell, Ranasinghe. The physiology of continuous-flow left ventricular assist devices, J Card Fail, 2017, Feb; 23(2) 169-180. [Online] URL: 10.1016/j.cardfail.2016.10.015
Additional Resources
- Royal Brompton and Harefield hospitals LVAD video patient information guides.
- Abbott healthcare patient information and education videos.
